Answer:
5.56g
Step-by-step explanation:
Expression of the equation:
2Mg + O₂ → 2MgO
Mass of MgO = 13.9g
Solution:
2Mg + O₂ → 2MgO
To solve this problem, let us find the number of moles of MgO from the given mas;
Number of moles =

molar mass of MgO = 24 + 16 = 40g/mol
Number of moles =
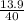 = 0.35mole
= 0.35mole
Now,
2 moles of MgO is produced from 1 mole of oxygen gas
0.35 mole of MgO will be produced from
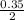 mole of oxygen gas
mole of oxygen gas
= 0.17mole of oxygen gas
So;
Mass of oxygen gas = number of moles x molar mass
Molar mass of O₂ = 2(16) = 32g/mol
Mass of oxygen gas = 0.17 x 32 = 5.56g