Answer:
3.97 x 10²³atoms
Step-by-step explanation:
Given parameters:
Mass of ammonia = 11.22g
Unknown:
Chemical amount of ammonia present = ?
Solution:
The chemical amount present in a substance is the number of atoms it contains.
To solve this problem, from the given mass find the number of moles first.
Number of moles =

Molar mass of NH₃ = 14 + 3(1) = 17g/mol
Number of moles =
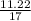 = 0.66mole
= 0.66mole
1 mole of any substance contains 6.02 x 10²³ atoms
0.66 mole of ammonia will contain 0.66 x 6.02 x 10²³ atoms
= 3.97 x 10²³atoms