Answer:
0.65mole of CO₂
Step-by-step explanation:
Given parameters:
Volume of CO₂ = 14.6L
Unknown:
Number of moles of CO₂ = ?
Solution:
To solve this problem, we must understand that a mole of a gas occupies a volume of 22.4L at STP;
1 mole of gas occupies 22.4L of volume at STP;
y mole of CO₂ will occupy 14.6L of volume at STP
22.4y = 14.6
y =
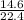 = 0.65mole of CO₂
= 0.65mole of CO₂