Answer:
107.9g
Step-by-step explanation:
Given parameters:
Molar mass of Cu = 63.5g/mol
Molar mass of Ag = 107.9g/mol
Mass of reacting Cu = 31.75g
Unknown:
Mass of Ag produced = ?
Solution:
The reaction equation is given as;
Cu + 2AgNO₃ → Cu(NO₃)₂ + 2Ag
The equation is balanced.
We start by find the number of moles of the known;
Number of moles =
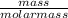
Number of moles of Cu =
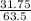 = 0.5moles
= 0.5moles
From the balanced equation;
1 mole of Cu produced 2 moles of Ag;
0.5 mole of cu will produce 0.5 x 2 mole of Ag = 1 mole of Ag
Now;
Mass of Ag = number of moles x molar mass
Mass of Ag = 1 mole of Ag x 107.9g/mol = 107.9g