Answer:
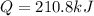
Step-by-step explanation:
Hello!
In this case, since the heat of vaporization is related with the energy required by a substance to undergo the phase transition from liquid to gas, we can compute such amount of energy as shown below:
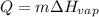
In such a way, since the enthalpy of vaporization is given as well as the mass, we compute the energy as shown below:
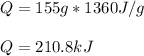
Best regards!