Answer:
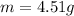
Step-by-step explanation:
Hello!
In this case, since the energy involved during a heating process is shown below:
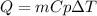
Whereas the specific heat of water is 4.184 J/(g°C), we can compute the heated mass of water by the addition of 11.9 kJ (11900 J) of heat as shown below:
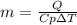
Thus, by plugging in, we obtain:
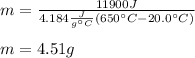
Best regards!