Given :
The pressure of 50.0 mL of oxygen gas at 100 ºC increases from 735 mm Hg to 925 mm Hg.
Temperature remains constant.
To Find :
The final volume.
Solution :
735 mm Hg = 0.967 atm
935 mm Hg = 1.230 atm
We know, at constant temperature :
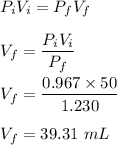
Therefore, final volume is 39.31 ml.
Hence, this is the required solution.