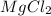Answer: The chemical formulas of calcium and magnesium chloride are
 and
and
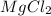
Step-by-step explanation:
A chemical formula is the representation of a substance in terms of chemical symbols showing the elements present in a compound and their relative proportion.
Calcium is having an atomic number of 20 has electronic configuration of
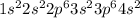 . It can lose two electrons to have an oxidation state of +2.
. It can lose two electrons to have an oxidation state of +2.
Magnesium is having an atomic number of 12 has electronic configuration of
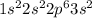 . It can lose two electrons to have an oxidation state of +2.
. It can lose two electrons to have an oxidation state of +2.
Chlorine is having an atomic number of 17 has electronic configuration of
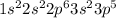 . It can gain one electron to have an oxidation state of -1.
. It can gain one electron to have an oxidation state of -1.
Thus the chemical formulas of calcium and magnesium chloride are
 and
and