Answer:
0.0013kg
Step-by-step explanation:
Given parameters:
Quantity of heat = 2.83kJ
Temperature = 100°C
Unknown:
Mass of the water = ?
Solution:
The amount of heat needed to vaporize a substance is dependent on the mass of the substance and its latent heat of vaporization.
H = mL
H is the amount of heat
m is the mass
L is the latent heat of vaporization
Latent heat of vaporization of water = 2260 kJ/kg
Now insert the parameters and solve;
2.83 = m x 2260
m =
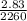 = 0.0013kg
= 0.0013kg