Answer:
0.23mol/kg
Step-by-step explanation:
Given parameters:
Mass of Potassium Bromide = 25g
Volume of pure water = 900mL
Unknown:
Molality = ?
Solution:
Molality is one of the ways of measuring concentration. It is the number of moles of solute in given mass of solvent.
Mathematically;
Molality =
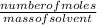
Number of moles of KBr;
Number of moles =

Molar mass of KBr = 39 + 80 = 119g/mol
Number of moles =
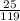 = 0.21mole
= 0.21mole
Mass of solvent;
Mass of solvent = density of water x volume of water
Mass of solvent = 1.0g/mL x 900mL = 900g = 0.9kg
Now;
Molality =
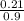 = 0.23mol/kg
= 0.23mol/kg