pH = 1.602
Further explanation
Ca(OH)₂+2HCl⇒CaCl₂+2H₂O
mol Ca(OH)₂ :
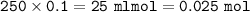
mol HCl :
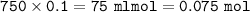
ICE method :
Ca(OH)₂+2HCl⇒CaCl₂+2H₂O
initial 0.025 0.075
change 0.025 0.05 0.025 0.05
equilbrium 0 0.025 0.025 0.05
There is a residual strong acid, then the pH is calculated from the acid concentration (H⁺)
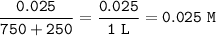
pH=-log [H⁺]
pH=-log[0.025]
pH=-log[2.5 x 10⁻²]
pH=2-log 2.5=1.602