Answer:
297.6 J
Explanation:
Given that,
Mass, m = 4 grams
We need to find how much heat is absorbed when 4.0 grams of steam is heated from 120°C to 160°C.
We know that heat absorbed is given by :
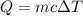
Where c is specific heat of water
Put all the values, we get :
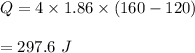
So, 297.6 J of heat is absorbed.