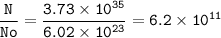The number of moles : 6.2 x 10¹¹
Further explanation
The mole is the number of particles contained in a substance
1 mole = 6.02.10²³ particles
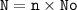
N = number of particles
n = moles
No = Avagadro's number : 6.02.10²³
3.73 x 10³⁵ atoms of S
the number of moles :