Answer:
7.3 x 10¹⁴Hz
Step-by-step explanation:
Given parameters:
Wavelength = 4.105 x 10⁻⁷m
Unknown:
Frequency of the light =?
Solution:
The frequency, wavelength and speed of a wave are related using the expression below;
C = f λ
C is the speed of light = 3 x 10⁸
f is the frequency
λ is the wavelength
3 x 10⁸ = f x 4.105 x 10⁻⁷
f =
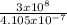 = 7.3 x 10¹⁴Hz
= 7.3 x 10¹⁴Hz