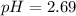Answer:
The correct option is d
Explanation:
From the question we are told that
The concentration of HF is [HF] = 0.6 M
The concentration of NaF is
![[NaF ] = 0.2 M](https://img.qammunity.org/2021/formulas/mathematics/college/jn1xm5vvziya3r7aejo9b8kqywf5c2mu5t.png)
The Ka of HF is
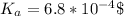
Generally HF(Hydrogen fluoride ) is ionized as follows
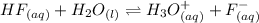
Generally NaF(Sodium fluoride ) is ionized as follows
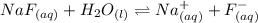
Generally the from Henderson-Hasselbalch equation the pH of the buffer is mathematically represented as
![pH = pKa + log [([NaF ])/(HF) ]](https://img.qammunity.org/2021/formulas/mathematics/college/1p2n49fb92dmwha652bdvddngbc6qwh603.png)
=>
![pH = -log(K_a) + log [([NaF ])/(HF) ]](https://img.qammunity.org/2021/formulas/mathematics/college/tn53dsalwxss2a24qk7nehs78t72e5bspy.png)
=>
![pH = -log(6.8 *10^(-4)) + log [([0.2 ])/(0.6) ]](https://img.qammunity.org/2021/formulas/mathematics/college/i95jg2ebvdjkg2qz3vfb9x8wsek9cxnkaf.png)
=>