Answer:
The question is incomplete. Searching in google I found: A 3.96x10⁻⁴ M solution of compound A exhibited an absorbance of 0.624 at 238 nm in a 1.000-cm cuvette. A blank solution containing only solvent had an absorbance of 0.029 at the same wavelength. Find the molar absorptivity of compound A.
The molar absorptivity of compound A is 1502.5 M⁻¹cm⁻¹.
Step-by-step explanation:
The question is incomplete. Searching in google I found: A 3.96x10⁻⁴ M solution of compound A exhibited an absorbance of 0.624 at 238 nm in a 1.000-cm cuvette. A blank solution containing only solvent had an absorbance of 0.029 at the same wavelength. Find the molar absorptivity of compound A.
The absorbance (A) is given by:
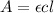
Where:
c: is the concentration of compound A = 3.96x10⁻⁴ M
ε: is the molar absorptivity of compound A =?
l: is the pathlength = 1 cm
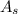 : is the absorbance of the solution= 0.624
: is the absorbance of the solution= 0.624
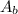 : is the absorbance of the blank solution= 0.029
: is the absorbance of the blank solution= 0.029
First, we need to find the absorbance of compound A:
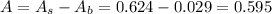
Now, we can calculate the molar absorptivity of compound A:
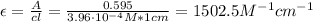
Therefore, the molar absorptivity of compound A is 1502.5 M⁻¹cm⁻¹.
I hope it helps you!