Answer:
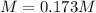
Step-by-step explanation:
Hello!
In this case, since the molarity of a solution is defined in terms of the moles of solute divided by the volume of the solution in liters and has units of mol/L or M:
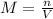
For the solute, Fe3Br2, which has a molar mass of 327.3430 g/mol, the moles in 42.389 g are:
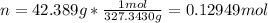
Thus, since the volume in liters is 0.750 L from those given 750 mL, the molarity turns out:
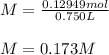
Best regards!