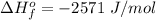Complete Question
10 g of Compound X with molecular formula
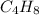 are burned in a constant-pressure calorimeter containing 45g of water at
are burned in a constant-pressure calorimeter containing 45g of water at
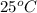 . The temperature of the water is observed to rise by 2.432. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound at
. The temperature of the water is observed to rise by 2.432. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound at
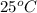
Answer:
The value is
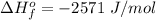
Step-by-step explanation:
From the question we are told that
The mass of compound X is
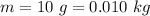
The mass of water is
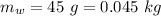
The temperature of water is
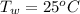
The change in the temperature of water is
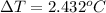
Generally the heat adsorbed by water is mathematically represented as
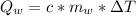
Here c is the specific heat of water with value
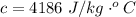
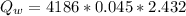
=>
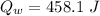
Given that the total heat that was generated by the reaction is absorbed by water then
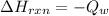
The negative sign shows that the heat was absorbed
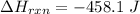
Generally the number of moles of the compound X available is mathematically represented as
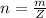
Here Z is the molar mass of compound X the value is
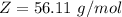
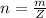
=>
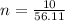
=>
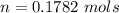
Generally the standard heat of formation of Compound X is mathematically represented as
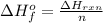
=>
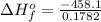
=>
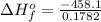
=>