Answer:
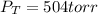
Step-by-step explanation:
Hello!
In this case, since the Dalton's law states that the total pressure inside a container full of various gases is equal to the addition of the pressure of each gas, for the given mixture containing nitrogen, oxygen and helium we have:
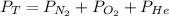
Thus, by plugging in each partial pressure, we obtain the total pressure of the mixture as shown below:
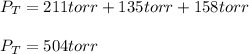
Best regards.