Answer:
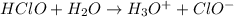
Step-by-step explanation:
Hello!
In this case, since hypochlorous acid (HClO) is a weak acid, which means it is not complete ionized in aqueous solution, represented by the reaction with water to obtain hydronium ions, which are the responsible of its acidity, and hypochlorite ions, we can write:
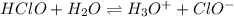
It is seen it is already balanced since there are three hydrogen atoms, one chlorine atom and two oxygen atoms at both reactants and products. It is important to notice the presence of the equilibrium arrow, which represents that the hydronium ions and the chlorate ions are able to come back to the initial hypochlorous acid and water.
Best regards!