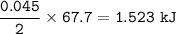Heat required : 1.523 kJ
Further explanation
Reaction
N₂+2O₂⇒2NO₂ AH = 67.7 kJ
mol of N₂:(use Pv=nRT)
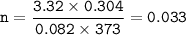
mol of O₂:
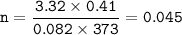
Limiting reactant :
N₂ : O₂
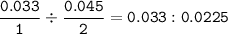
Limiting reactant : O₂(smaller ratio)
mol NO₂ = mol O₂ = 0.045
2 mol ⇒ 67.7 kJ, so for 0.045 mol, heat required :