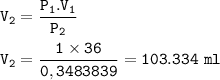The new volume of the gas : 103.334 ml
Further explanation
Boyle's Law
At a constant temperature, the gas volume is inversely proportional to the pressure applied
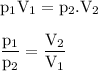
1 kPa = 0,00986923 atm
35.3 kPa = 0,3483839 atm
at STP ( 0°C, 1 atm) with volume 36 ml changed to 35.3 kPa :
P₁=1 atm
V₁=36 ml
P₂= 0,3483839 atm
The new volume(V₂) :