Answer:
A. Convert the 200 mol of water to kilograms of water.
Step-by-step explanation:
To find the molality of 10mol of NaCl in 200mol of water, convert the 200mol of water to kilograms of water.
Molality is one of the several ways of measuring concentration of a solute in a solvent;
Molality =
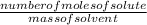
- To solve this problem, simply convert the 200mole to mass of water.