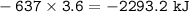1. HNO₃ (g) + 4H₂ (g) ⇒ NH₃ (g) + 3H₂O (g)
2. Exothermic.
3. 2293.2 kJ
Further explanation
Reaction
HNO₃ (g) + H₂ (g) ⇒ NH₃ (g) + H₂O (g) △H = −637 kJ
1. Balance
give coefficient :
HNO₃ (g) + aH₂ (g) ⇒ bNH₃ (g) + cH₂O (g)
H, left=1+2a, right=3b+2c⇒1+2a=3b+2c(eq 1)
N,left=1, right=b⇒b=1
O,left=3, right=c⇒c=3
eq 1 : 1+2a=3.1+2.3⇒1+2a=9⇒2a=8⇒a=4
Balance reaction :
HNO₃ (g) + 4H₂ (g) ⇒ NH₃ (g) + 3H₂O (g)
2. exothermic⇒release heat(negative sign)
3. heat released :
mol H₂ :
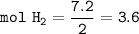
heat released :