a. The symbol
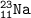 ,
,
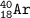 ,
,
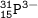 ,
,
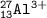
b. Isotope of Na
Further explanation
Atomic number=number of protons=number of electrons(neutral atom)
Number of neutrons=mass number-atomic number
Mass number=protons+neutrons
B. atomic number=11, mass number=11+12=23⇒Sodium
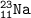
C. atomic number=18, mass number=18+22=40⇒Argon
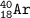
D. atomic number=15, mass number=15+16=31, charge -3(electron-proton)⇒Phosphor
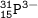
E. atomic number=13, mass number=13+14=27, charge +3(proton-electron)⇒Aluminium
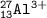
Isotope=the same atomic mass, different neutron(mass number)
So the atom⇒isotope of Sodium(Na)