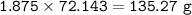Mass of CaS : 135.27 g
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction
4 HgS(s)+ 4 CaO(s) → 3 CaS(s) + CaSO₄(s) + 4Hg(l).
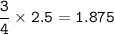
MW CaS : 72,143 g/mol