Answer:
25.9 g
Step-by-step explanation:
Given that,
No of moles of calcium phosphate,
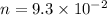
We need to find how many grams of calcium phosphate has this much of no of moles.
Mass divided by molar mass is equal to the no of moles on a molecule.
The molar mass of calcium phosphate is 310.18 g/mol
Using the concept of no of moles as follows :
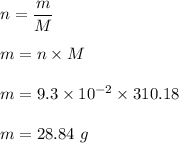
Out of given options, option (a) i.e. 25.9 g is the correct answer.