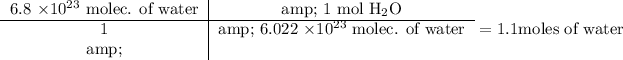Answer:
Our final conversion is 1.1 moles of water.
Step-by-step explanation:
When we want to find the amount of moles in a substance, we can use dimensional analysis or conversion factors in order to convert it from molecules, grams, atoms, formula units, or virtually any other unit.
We are given that we want to convert 6.8 x 10²³ molecules of water to moles. We can set up a dimensional analysis table to solve this.
In dimensional analysis, we multiply across the top and then divide the results by the multiplied value on the bottom.
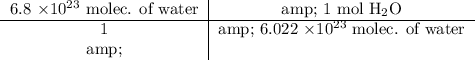
Now, we will perform our calculations and find our final answer.
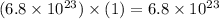
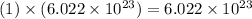
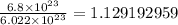
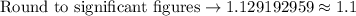
Therefore, using our table, we have determined that our final answer is 1.1 moles of water.