Answer:
24 mol Cu
General Formulas and Concepts:
Chemistry
Step-by-step explanation:
Step 1: Define
RxN: 2Cu (s) + O₂ (g) → 2CuO (s)
Given: 12 moles O₂
Step 2: Stoichiometry
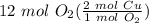 = 24 mol Cu
= 24 mol Cu
Step 3: Check
We are given 2 sig figs.
Our final answer is in 2 sig figs, so no need to round.