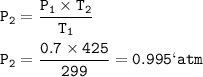The pressure of the CO₂ = 0.995 atm
Further explanation
The complete question
A student is doing experiments with CO2(g). Originally, a sample of gas is in a rigid container at 299K and 0.70 atm. The student increases the temperature of the CO2(g) in the container to 425K.
Calculate the pressure of the CO₂ (g) in the container at 425 K.
Gay Lussac's Law
When the volume is not changed, the gas pressure is proportional to its absolute temperature
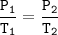
P₁=0.7 atm
T₁=299 K
T₂=425 K