Answer:
0.625mol/L
Step-by-step explanation:
Given parameters:
Number of moles of solute = 5moles
Volume of solvent = 8L
Unknown:
Molarity = ?
Solution:
Molarity is the number of moles of solute per unit volume of solution. It is mathematically expressed as
Molarity =
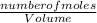
Molarity =
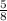 = 0.625mol/L
= 0.625mol/L