Answer:
0.08kg
Step-by-step explanation:
Given parameters:
Temperature of water = 100°C
Quantity of heat released = 180414.08J
Unknown:
Quantity of water condensed = ?
Solution:
This is simple phase change process without any attendant change in temperature.
To solve this problem, use the formula below;
H = mL
H is the heat released
m is mass
L is the latent heat of condensation of water = 2260 kJ/kg
Insert the parameters and solve;
180,414.08 = m x 22.6 x 10⁵ J/kg
m =
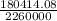 = 0.08kg
= 0.08kg