Answer:
0.02kg
Step-by-step explanation:
Given parameters:
Amount of heat = 5200J
Unknown:
Mass of ice that would be melted at 0°C = ?
Solution:
To solve this problem, use the expression below;
H = mL
H is the heat
m is the mass
L is the latent heat of fusion of ice = 3.33 x 10⁵ J/kg.
Insert the parameters and solve for m;
5200 = m x 3.3 x 10⁵
m =
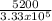 = 0.02kg
= 0.02kg