We are given:
Mass of the tablet = 2.7 grams
Number of moles of Ascorbic acid present in the tablet = 0.0109 moles
Molar mass of Ascorbic acid = 176.12 g/mol
Mass of Ascorbic Acid present in the tablet:
Mass of a given number of moles = number of moles * molar mass
replacing the variables
Mass = 0.0109 * 176.12
Mass = 1.92 grams (approx)
Mass percent of Ascorbic acid:
Mass% = (
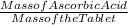 ) * 100
) * 100
replacing the variables
Mass% = (1.92 / 2.7) * 100
Mass% = 192 / 2.7
Mass% = 71.12 %
Therefore, the tablet has 71.12% Ascorbic Acid by mass