Answer:
4 moles of N₂O₅
Step-by-step explanation:
Given parameters:
Number of moles of O₂ = 10moles
Unknown:
Number of moles of N₂O₅ produced = ?
Solution:
To solve this problem, we must write the reaction equation first,
2N₂ + 5O₂ → 2N₂O₅
IN the reaction above;
5 moles of O₂ will produce 2 moles of N₂O₅;
10 mole of O₂ will produce
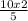 = 4 moles of N₂O₅
= 4 moles of N₂O₅