Answer:
11.9 g S
General Formulas and Concepts:
Chem
- Reading a Periodic Table
- Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
- Dimensional Analysis
Step-by-step explanation:
Step 1: Define
2.23 × 10²³ atoms S
Step 2: Define conversions
Avogadro's Number
Molar Mass of S - 32.07 g/mol
Step 3: Dimensional Analysis
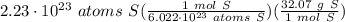 = 11.8758 g S
= 11.8758 g S
Step 4: Check
We are given 3 sig figs. Follow sig fig rules.
11.8758 g S ≈ 11.9 g S