Answer:
20 mol H₂
General Formulas and Concepts:
Chem
Step-by-step explanation:
Step 1: Define
RxN: C + H₂ → CH₄
8 moles CH₄
Step 2: Balance RxN
C + 2H₂ → CH₄
Step 3: Stoichiometry
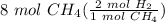 = 16 mol H₂
= 16 mol H₂
Step 4: Check
We are given 1 sig fig. Follow sig fig rules.
16 mol H₂ ≈ 20 mol H₂