Answer:
The fourth option
Step-by-step explanation:
So if you got the periodic table, you can search for boron and get it's Molar Mass: B=10.811u
We know the given mass and we know that there's only 1 mol of boron given, we can calculate it by the given:
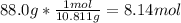
We use avogadro's number (6.022×10²²), plug in our new value to get the # atoms:
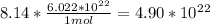 (Multiply 8.14 by Avogadro's number to get the atom number)
(Multiply 8.14 by Avogadro's number to get the atom number)
so the atom number for 88g of Boron would be : 4.90×10²² atoms of B