Answer:
A single compound is produced in a synthesis reaction.
Step-by-step explanation:
What are chemical reactions?
Chemical reactions are when two or more reactants chemically react with one another to create one or more substances as products. There are five types: synthesis, decomposition, single-replacement, double-replacement, and combustion.
Types of Reactions and Examples of Each
A synthesis reaction takes two or more reactants and reacts chemically to turn them all into one substance.
- An example of a synthesis reaction is the reaction that takes place between sodium (Na) and chlorine (Cl) to create table salt.
- This reaction would be displayed as
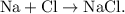
- The parent reaction for a synthesis reaction is
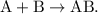
A decomposition reaction takes one reactant and breaks itself into two or more products.
- An example of a decomposition reaction would be the decomposition of hydrogen (H₂) and oxygen (O) to create two diatomic molecules - H₂ + O₂.
- This reaction would be displayed as
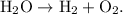
- The parent reaction for a decomposition reaction is
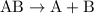 .
.
A single-replacement reaction takes place when two compounds chemically combine but one of the elements bonds with a different element.
- An example of a single-replacement reaction would be the single-replacement of calcium and water to create calcium hydroxide and dihydrogen.
- This reaction would be displayed as
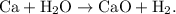
- The parent reaction for a single-replacement reaction is
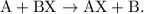
A double-replacement reaction works in the same way as a single-replacement reaction - however, instead of one replacement, there are two replacements.
- An example of a double-replacement reaction would be the double-replacement of sodium bicarbonate (baking soda) and vinegar to produce carbonic acid and sodium acetate.
- This reaction would be displayed as
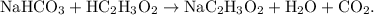
- The parent reaction for a double-replacement reaction is
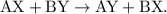
A combustion reaction is the introduction of heat or energy and oxygen to decompose reactants into carbon dioxide and water.
- An example of a combustion reaction would be the burning of propane in a grill.
- This reaction would be displayed as
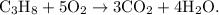
- The parent reaction for a combustion reaction is
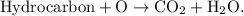
- A hydrocarbon is a substance that is only hydrogen and carbon.
Therefore, based on the above information, we can determine that a single compound, or option B, is the product of a synthesis reaction.