Answer:
the 4s subshell is lower in energy than the 3d subshell
Step-by-step explanation:
In the element number 24 which is chromium
the electronic configuration should be according to Aufbau principle
will be =
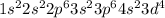
but in reality it is =
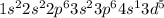
The 6 electrons in the outermost shell will be divided as 5 in 3d subshell and 1 in 4s shell, because half filled orbital
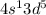 will be more stable than
will be more stable than
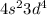 .
.