Answer:
Step-by-step explanation:
- The atomic mass of carbon is 12.011 g/mol.
- The atomic mass of hydrogen is 1.00794 g/mol.
This means that the atomic mass of methane is 12.011+4(1.00794)=16.04276 g/mol.
- The percent composition by mass of carbon is
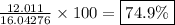
- The percent composition by mass of hydrogen is
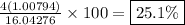
This means that in a 37.8-gram sample of methane, there would be
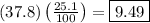 grams of hydrogen.
grams of hydrogen.