Answer:
c. 131 kPa
Step-by-step explanation:
Hello!
In this case, since the relationship between volume and pressure is inversely proportional, based on the Boyle's law:

Considering that the standard pressure is 101.325 kPa, we can compute the final pressure as shown below:
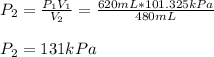
Therefore, the answer is c. 131 kPa .
Best regards!!