Answer:
6.02 x 10²³ electrons
Step-by-step explanation:
Given parameters:
Mass of H₂ = 2g
Unknown:
Number of electrons = ?
Solution:
To find the number of electrons, we must determine the number of moles of H₂ first.
Number of moles =

Molar mass of H₂ = 2(1) = 2g/mol
Number of moles =
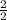 = 1mol
= 1mol
1 mole of a substance contains 6.02 x 10²³ particles
the particles can be protons, neutrons, electrons
So,
2g of H₂ will contain 6.02 x 10²³ electrons.