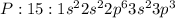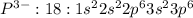Answer: anion , gained
Step-by-step explanation:
When an atom accepts an electron negative charge is created on atom and is called as anion.
When atom loses an electron positive charge is created on atom and is called as cation.
Phosphorous (P) has an atomic number of 15 which means it contains 15 electrons and there are 5 valence electrons.
For formation of , the atom of phosphorous (P) must gain three electrons in its valence shell 3p so as to complete its octet