Answer:
34672.96 s
Step-by-step explanation:
m = Mass of copper = 6.32 g
M = Molar mass of copper = 63.5 g/mol
F = Faraday constant = 96500 C/mol
The electrode equation would be
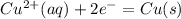
Number of electrons = 2 = e
I = Current = 0.554 A
t = Time taken
Charge would be
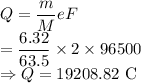
Charge is given by
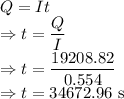
Time taken to deposit the copper is 34672.96 s.