Answer:
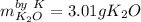
Step-by-step explanation:
Hello!
In this case, since 2.50 g of both potassium (molar mass = 39.1 g/mol) and gaseous oxygen (molar mass = 32.0 g/mol) react in a 4:1 and 1:2 mole ratio respectively, to produce potassium oxide (molar mass = 94.2 g/mol), we evaluate the mass of potassium oxide yielded by each reactant in order to identify the limiting one via stoichiometry:
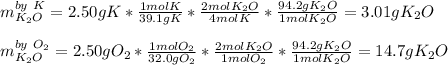
Thus, since the 2.50 g of potassium yields 3.01 g of potassium oxide, we infer it is the limiting reactant and that is the mass of produced product by the reaction.
Best regards!