Answer:
29 mL HCl
General Formulas and Concepts:
Chem
- Molarity = moles of solute / liters of solution
Step-by-step explanation:
Step 1: Define
6.2 M HCl
0.18 mol HCl
x L mol HCl
Step 2: Define conversions
1 L = 1000 mL
Step 3: Find L
6.2 M HCl = 0.18 mol HCl / x L HCl
(x L HCl)(6.2 M HCl) = 0.18 mol HCl
x L HCl = 0.18 mol HCl / 6.2 M HCl
x = 0.029032 L HCl
Step 4: Convert
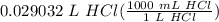 = 29.0323 mL HCl
= 29.0323 mL HCl
Step 5: Check
We are given 2 sig figs. Follow sig fig rules.
29.0323 mL HCl ≈ 29 mL HCl