Answer:
The original concentration of the HCl is: 0.01925 M
Step-by-step explanation:
Equation:
HCl+NaOH-------> NaCl+H₂O
Volume of NaOH added = 0.05 M
No of moles of NaOH = 15.4 mL
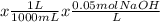
= 0.00077 mol NaOH
Then,
Volume of HCl solution = 40 mL x 1/1000 mL
= 0.0400 L
Therefore,
Concentration of HCl = 0.00077 mol/0.0400 L
= 0.01925 M
Now, to find the pH:
pH = -log₁₀[H⁺]
= -log₁₀(2x10⁻⁶)
= 5.7