Answer:
0.014mol
Step-by-step explanation:
Given parameters:
Mass of Na₂CO₃ = 1.5g
Unknown:
Number of moles = ?
Solution:
Number of moles of a compound is mathematically expressed as;
Number of moles =

Molar mass of Na₂CO₃ = 2(23) + 12 + 3(16) = 106g/mol
Number of moles =
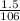 = 0.014mol
= 0.014mol