Answer:
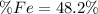
Step-by-step explanation:
Hello!
In this case, since the percent composition of an element in a compound is given by:
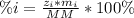
Whereas i represent the element in the compound for which we want compute the mass percent. As required for siderite, FeCO3 whose molar mass is 115.854 g/mol, and knowing there is one iron atom there with an atomic mass od 55.85 g/mol, the mass percent of iron there is:
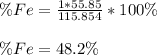
Best regards!